BLOG
What is Epigenomics?
Epigenomics is different than epigenetics and it’s not simply a matter of scale. As I explained in my previous post, epigenetics, like genetics, is concerned with matters of inheritance. In contrast, epigenomics is the study of those factors that form chromatin, regardless of whether they are inherited. Chromatin roughly refers to those factors that interact with DNA and determine how it is “packaged” into its overall structure and it includes things like DNA methylation, histones, histone modifications, and non-coding RNAs. The study of chromatin goes back a long way, predating epigenomics. The term “chromatin” was first coined by Walther Flemming in the late 1870s & early 1880s who noticed that structures within cells that would stain with certain dyes, hence “chromatin” or “stainable material” [1]. What makes epigenomics unique, however, is the use of modern genomics techniques like microarrays or high-throughput sequencing methods to study these factors at high resolution across the entire genome.
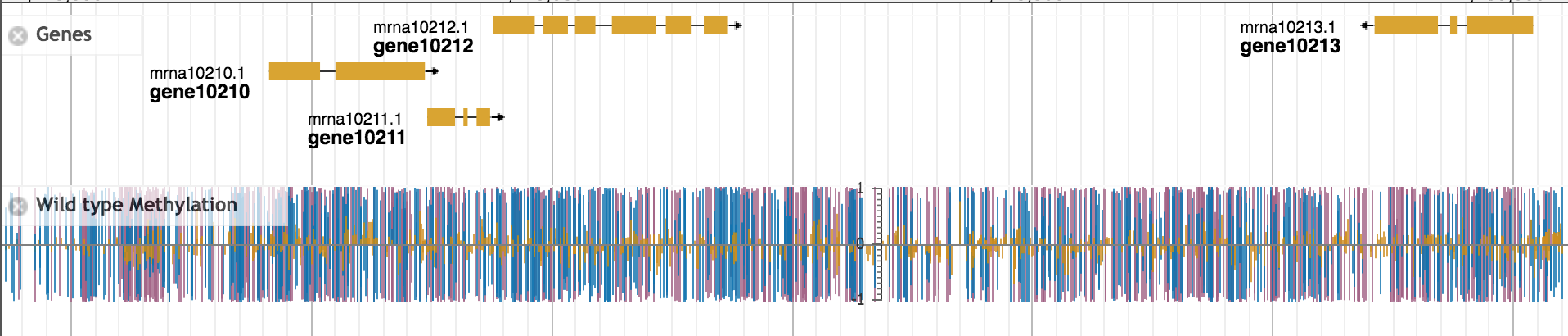 Figure 1: DNA methylation in the diploid strawberry species Fragaria vesca
Figure 1: DNA methylation in the diploid strawberry species Fragaria vesca
This is approach is incredibly powerful, as it allows us to know study factors like DNA methylation or various histone modifications at “single-base resolution”. In other words, we can now identify specifically every cytosine in the genome that is methylated or unmethylated or every gene targeted by a given histone modification. We can then integrate this information with other types of data, such as gene expression data or phenotypic data to understand how these chromatin factors are involved in development, environmental responses, or even the evolution of the organism.
While in some circumstances, these chromatin factors may be stably transmitted to new cells or even new generations (epigenetics), this is not always the case. The epigenome can sometimes dynamically change in response to a stimulus and then change again without ever passing onto the next generation. In such cases, the epigenome is not being “inherited” and so is not “epigenetic”. But it’s still considered to be part of the “epigenome”. Confusing, right? As I illustrated in my post on epigenetics, there is a degree of overlap between epigenetics and epigenomics, but there are also aspects of each that are not part of the other. That’s why it’s important for us to be careful to distinguish between the two.
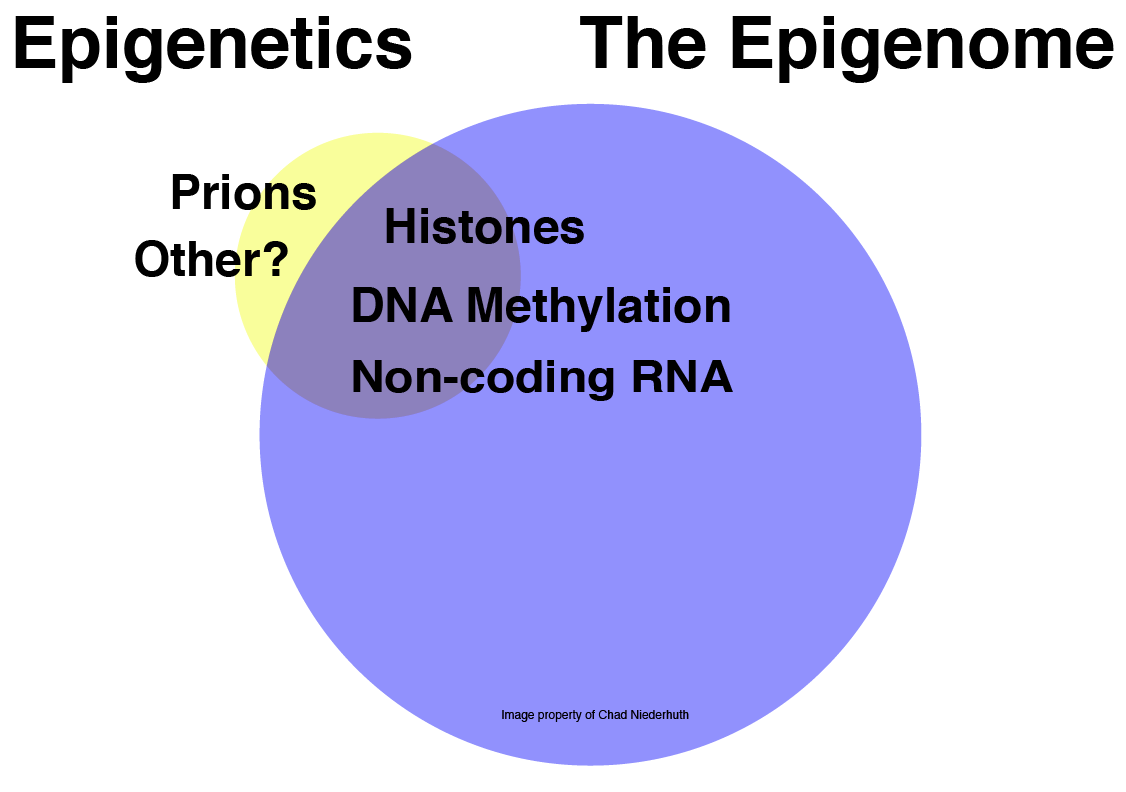 Figure 2: Mechanistic relationship between Epigenetics and Epigenomics. Note that the area and overlap of the venn diagram is not based on any actual data, it’s just to illustrate a concept.
Figure 2: Mechanistic relationship between Epigenetics and Epigenomics. Note that the area and overlap of the venn diagram is not based on any actual data, it’s just to illustrate a concept.
In the Niederhuth lab the bulk of the effort is in trying to understand the epigenome and its function. As these factors are important to the overall regulation of the genome, this can help us better understand how a plant’s DNA makes a plant and ultimately how we can use this knowledge to improve plants. There are many ways to understanding epigenome function, but oftentimes the best way is to let nature guide us and look at the differences and similarity between individuals and species that already exist. Such approaches include “population epigenomics” and “comparative epigenomics”, which look at the epigenome in the context of a population of individuals of the same species (population epigenomics) or which compares the epigenomes of different species (comparative epigenomics). Using these approaches has already led us to understanding many of the factors that create such differences over evolutionary time (This will be a topic of some future posts) [2,3]. Longer term, they will hopefully lead to the identification of useful variation that could even be applied for crop improvement [4].
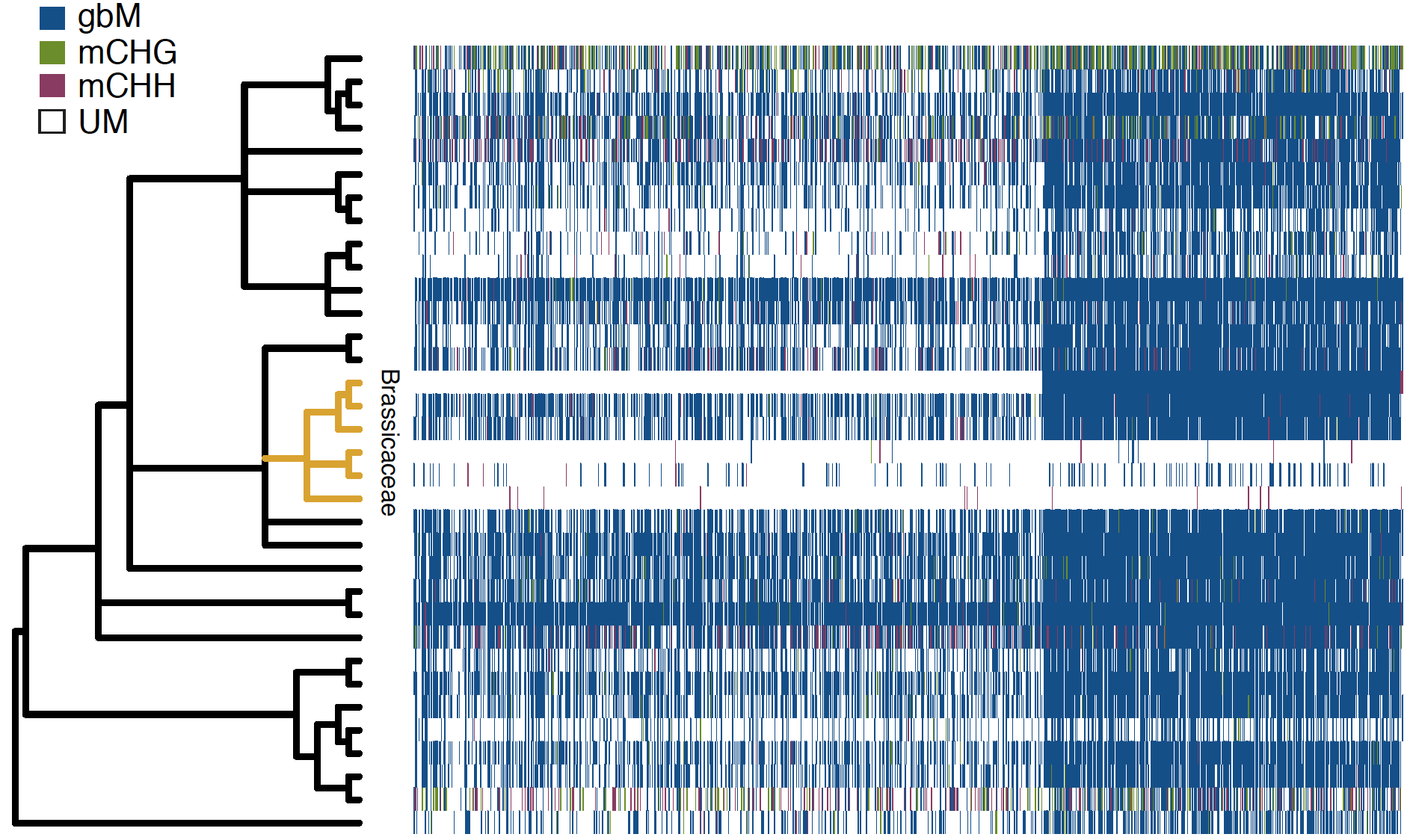 Figure 3: Heatmap showing methylation state of orthologous genes across 34 different plant species. Each column is an orthologous gene and each row is a species. Source (Niederhuth et al, 2016)
Figure 3: Heatmap showing methylation state of orthologous genes across 34 different plant species. Each column is an orthologous gene and each row is a species. Source (Niederhuth et al, 2016)
Written by Chad Niederhuth date: 2017-11-08T13:54:34-08:00
References
Paweletz, N., 2001 Walther Flemming: pioneer of mitosis research. Nat Rev Mol Cell Biol 2: 72-75.
Niederhuth, C. E., A. J. Bewick, L. Ji, M. S. Alabady, K. D. Kim et al., 2016 Widespread natural variation of DNA methylation within angiosperms. Genome Biol 17: 194.
Bewick, A. J., L. Ji, C. E. Niederhuth, E. M. Willing, B. T. Hofmeister et al., 2016 On the origin and evolutionary consequences of gene body DNA methylation. Proc Natl Acad Sci U S A 113: 9111-9116.
Ji, L., D. A. Neumann and R. J. Schmitz, 2015 Crop Epigenomics: Identifying, Unlocking, and Harnessing Cryptic Variation in Crop Genomes. Mol Plant 8: 860-870.